Services



Source: Global Observatory on Health R&D (2022).
Source: The Global Competitiveness Report (2019).
Source: 2022 indicators data
Source: 2022 indicators data

A specialised team, beyond Science
The Portugal Clinical Studies is a platform by the Clinical Research and Biomedical Innovation Agency that brings together a dynamic and specialized team to help streamline and expedite the processes inherent to your clinical study.

Why Portugal?
Positioning Portugal among the most attractive countries for conducting clinical trials in the European Union is the vision of the Portuguese government for the field of clinical research. Clinical trials are one of the main strategic areas for the development of healthcare in Portugal.
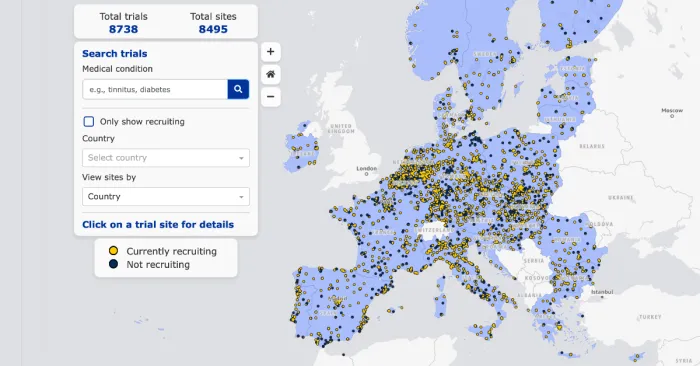
New Map of Clinical Trials launched in the EU
The Clinical Trials Information System (CTIS)️ launched the new interactive map that facilitates access to information about clinical trials in the European Union on March 3, 2025.
This map allows users to search for ongoing Clinical Trials (CT) by location and medical condition, as well as access investigator contact details to learn how to participate in the CT.
Clinical Research Sites (CRSs)
The CRSs, also known as Clinical Research Sites, drive comprehensive clinical research, ranging from study development to professional training, promoting collaboration and excellence in the healthcare field.